Exam 16: Acids and Bases
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Carbon dioxide combines with rainwater to form
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
Identify the acid that is in car batteries.
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
Which of the following is an Arrhenius base?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
B
Calculate the hydronium ion concentration in an aqueous solution with a pOH of 9.85 at 25°C.
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following solutions would have the highest pH? Assume that they are all 0.10 M in acid at 25∘C.The acid is followed by its Ka value.
(Multiple Choice)
4.9/5  (39)
(39)
A solution with a hydrogen ion concentration of 3.25 × 10-6 M is ________ and has a hydroxide ion concentration of ________.
(Multiple Choice)
4.8/5  (26)
(26)
Find the percent ionization of a 0.337 M HF solution.The Ka for HF is 3.5 × 10-4.
(Multiple Choice)
4.8/5  (38)
(38)
What is the pH of pure water at 40.0°C if the Kw at this temperature is 2.92 × 10-14?
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the concentration of bicarbonate ion,HCO3-,in a 0.010 M H2CO3 solution that has the stepwise dissociation constants Ka1 = 4.3 × 10-7 and Ka2 = 5.6 × 10-11.
(Multiple Choice)
4.8/5  (39)
(39)
Which Br∅nsted-Lowry acid is NOT considered to be a strong acid in water?
(Multiple Choice)
4.8/5  (42)
(42)
Determine the [H3O⁺] in a 0.265 M HClO solution.The Ka of HClO is 2.9 × 10-8.
(Multiple Choice)
4.8/5  (32)
(32)
Which one of the following will form an acidic solution in water?
(Multiple Choice)
4.8/5  (48)
(48)
Which of the following bases is the strongest? The base is followed by its Kb value.
(Multiple Choice)
4.9/5  (40)
(40)
Determine the pH of a 0.461 M C6H5CO2H M solution if the Ka of C6H5CO2H is 6.5 × 10-5.
(Multiple Choice)
4.8/5  (39)
(39)
What is the pH of a 0.30 M pyridine solution that has Kb = 1.9 × 10-9? The equation for the dissociation of pyridine is: C5H5N(aq)+ H2O(l)⇌ C5H5NH+(aq)+ OH-(aq)
(Multiple Choice)
4.9/5  (42)
(42)
Calculate the pH of a 1.60 M KBrO solution.Ka for hypobromous acid,HBrO,is 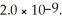
(Multiple Choice)
4.9/5  (31)
(31)
Determine the concentration of CO32- ions in a 0.18 M H2CO3 solution.Carbonic acid is a diprotic acid whose Ka1 = 4.3 × 10-7 and Ka2 = 5.6 × 10-11.
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 160
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)