Exam 4: Chemical Quantities and Aqueous Reactions
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Which of the following is considered a STRONG electrolyte?
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
A
How many of the following compounds are soluble in water? Pb(OH)2 LiNO3 NH4Br K2S
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
D
What reagent could NOT be used to separate OH- from Cl- when added to an aqueous solution containing both?
(Multiple Choice)
4.9/5  (30)
(30)
A solution is prepared by mixing 5.00 mL of 0.100 M HCl and 2.00 mL of 0.200 M NaCl.What is the molarity of chloride ion in this solution?
(Multiple Choice)
4.7/5  (35)
(35)
Lithium and nitrogen react to produce lithium nitride: 6 Li(s)+ 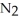 (g)→ 2 Li3N(s)
How many moles of lithium nitride are produced when 0.750 mol of lithium react in this fashion?
(g)→ 2 Li3N(s)
How many moles of lithium nitride are produced when 0.750 mol of lithium react in this fashion?
(Multiple Choice)
4.9/5  (33)
(33)
Identify the oxidation state of Ca in CaF2(aq). Ca(s)+ 2 HF(aq)→ CaF2(aq)+ H2(g)
(Multiple Choice)
4.8/5  (34)
(34)
Give the complete ionic equation for the reaction (if any)that occurs when aqueous solutions of MgSO3 and HI are mixed.
(Multiple Choice)
4.8/5  (31)
(31)
How many carbonate ions are present in 30.0 mL of 0.600 M Li2CO3 solution?
(Multiple Choice)
4.7/5  (42)
(42)
Determine the concentration of a solution prepared by diluting 20.0 mL of a 0.200 M LiCl to 250.0 mL.
(Multiple Choice)
4.9/5  (29)
(29)
Consider the following balanced reaction.How many grams of water are required to form 75.9 g of HNO3? Assume that there is excess NO2 present.The molar masses are as follows: H2O = 18.02 g/mol,HNO3 = 63.02 g/mol. 3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
(Multiple Choice)
4.8/5  (31)
(31)
Identify the oxidizing agent. 2 Al3+(aq)+ 2 Fe(s)→ 2 Al(s)+ 3 Fe2+(aq)
(Multiple Choice)
4.7/5  (25)
(25)
If the percent yield for the following reaction is 65.0%,how many grams of KClO3 are needed to produce 4.00 g of O2? 2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
(Multiple Choice)
4.8/5  (30)
(30)
How many milliliters of a stock solution of 13.5 M HNO3 would be needed to prepare 0.500 L of 0.500 M HNO3?
(Multiple Choice)
4.9/5  (35)
(35)
The density of ethanol,C2H5OH,is 0.789 g/mL.How many milliliters of ethanol are needed to produce 30.0 g of CO2 according to the following chemical equation? C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(l)
(Multiple Choice)
4.7/5  (48)
(48)
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6 Li(s)+ N2(g)→ 2 Li3N(s)
In a particular experiment,1.50-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 239
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)