Exam 10: Organic Chemistry
Exam 1: Temperature and Heat121 Questions
Exam 2: Waves and Sound96 Questions
Exam 3: Optics and Wave Effects105 Questions
Exam 4: Electricity and Magnetism106 Questions
Exam 5: Atomic Physics105 Questions
Exam 6: Nuclear Physics107 Questions
Exam 7: The Chemical Elements101 Questions
Exam 8: Chemical Bonding109 Questions
Exam 9: Chemical Reactions170 Questions
Exam 10: Organic Chemistry169 Questions
Exam 11: Place and Time179 Questions
Exam 13: The Solar System143 Questions
Exam 15: Moons and Small Solar System Bodies147 Questions
Exam 16: The Universe104 Questions
Exam 17: The Atmosphere152 Questions
Exam 19: Atmospheric Effects101 Questions
Exam 21: Structural Geology and Plate Tectonics161 Questions
Exam 22: Minerals Rocks and Volcanoes170 Questions
Exam 23: Surface Processes121 Questions
Exam 24: Geologic Time149 Questions
Select questions type
The collective name for neutrons and protons is ______________.
Free
(Short Answer)
4.8/5  (38)
(38)
Correct Answer:
nucleons
No nuclide with an atomic number greater than ______________ is stable.
Free
(Short Answer)
4.9/5  (40)
(40)
Correct Answer:
83
How many protons are there in an atom of 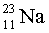 ?
?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
D
The half-life of 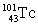 is 14 min. How many minutes would it take for the activity of a sample of this radionuclide to decrease from 1200 cpm to 150 cpm?
is 14 min. How many minutes would it take for the activity of a sample of this radionuclide to decrease from 1200 cpm to 150 cpm?
(Short Answer)
4.7/5  (23)
(23)
When an oxygen-19 nucleus undergoes beta decay, the nucleus formed is that of
(Multiple Choice)
4.9/5  (28)
(28)
Short-term and long-term effects of radiation on the health of the recipient are called ______________ effects.
(Multiple Choice)
4.7/5  (28)
(28)
Short-term and long-term effects of radiation on the health of the recipient are called ______________ effects.
(Short Answer)
4.9/5  (29)
(29)
The atomic mass of a hypothetical element consisting of 50% 30X and 50% 32X would be about ______________ u.
(Short Answer)
4.9/5  (37)
(37)
With only two exceptions, if a nucleus contains fewer ______________ than ______________, it is unstable.
(Multiple Choice)
4.9/5  (29)
(29)
A fusion reaction occurring in the interior of some stars is shown below. Calculate the mass lost, in atomic mass units, and the energy produced, in millions of electron volts. (Recall that 931 MeV is the energy obtained from the conversion of 1 u of mass.)
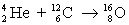 4.00260 u 12.0000 u 15.9949 u
4.00260 u 12.0000 u 15.9949 u
(Short Answer)
4.8/5  (34)
(34)
When an unstable Ag nucleus undergoes gamma decay, the nucleus formed is that of
(Multiple Choice)
4.7/5  (38)
(38)
Only the energy of a nucleus, and not its identity, is changed when it undergoes ______________ decay.
(Short Answer)
4.8/5  (33)
(33)
Showing 1 - 20 of 169
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)