Exam 5: Atomic Physics
Exam 1: Temperature and Heat121 Questions
Exam 2: Waves and Sound96 Questions
Exam 3: Optics and Wave Effects105 Questions
Exam 4: Electricity and Magnetism106 Questions
Exam 5: Atomic Physics105 Questions
Exam 6: Nuclear Physics107 Questions
Exam 7: The Chemical Elements101 Questions
Exam 8: Chemical Bonding109 Questions
Exam 9: Chemical Reactions170 Questions
Exam 10: Organic Chemistry169 Questions
Exam 11: Place and Time179 Questions
Exam 13: The Solar System143 Questions
Exam 15: Moons and Small Solar System Bodies147 Questions
Exam 16: The Universe104 Questions
Exam 17: The Atmosphere152 Questions
Exam 19: Atmospheric Effects101 Questions
Exam 21: Structural Geology and Plate Tectonics161 Questions
Exam 22: Minerals Rocks and Volcanoes170 Questions
Exam 23: Surface Processes121 Questions
Exam 24: Geologic Time149 Questions
Select questions type
The piston of a cylinder containing a quantity of ideal gas is advanced so that the volume of the gas is decreased by one-half. A pressure gauge on the cylinder shows the pressure of the gas to have increased threefold in the process. By what factor does the temperature of the gas change?
Free
(Essay)
4.8/5  (40)
(40)
Correct Answer:
It increases by a factor of 3/2 (T2 = 3/2 T1).
The amount of heat necessary to change a liquid to a solid at constant temperature is the ______________.
Free
(Short Answer)
4.8/5  (37)
(37)
Correct Answer:
latent heat of fusion
On bare feet, a tile floor feels colder than a rug because the tile has a greater ______________.
Free
(Short Answer)
4.9/5  (34)
(34)
Correct Answer:
thermal conductivity
On a winter day the temperature drops from -5°C to -15°C overnight. If a pan sitting outside contains 0.40 kg of ice, how much heat is removed from the ice for this temperature change?
(Short Answer)
4.9/5  (38)
(38)
What happens to a sample of water when its temperature is reduced between 4°C and 100°C?
(Multiple Choice)
4.7/5  (29)
(29)
Heat transfer from hot or cold liquids in a thermos bottle is prevented by ______________.
(Multiple Choice)
4.9/5  (41)
(41)
In measuring any temperature less than 500 Fahrenheit, which of the following scales will have the highest numeric reading?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following has the highest thermal conductivity?
(Multiple Choice)
4.8/5  (31)
(31)
How much heat is required to melt 5.0 kg of ice at 0°C to water at 0°C?
(Essay)
4.8/5  (38)
(38)
It is 23°F outside.a. What is the Celsius temperature?
b. If this Fahrenheit temperature were doubled, what would the new Celsius temperature be?
(Short Answer)
4.9/5  (35)
(35)
The amount of heat necessary to change 1 kg of a solid into a liquid at the same temperature is called the
(Multiple Choice)
4.8/5  (33)
(33)
What happens to a sample of water when it is heated between 4°C and 100°C?
(Multiple Choice)
4.8/5  (38)
(38)
Use the following to answer questions :
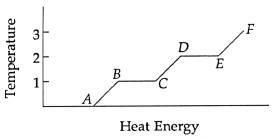 -The latent heat of fusion is the amount of energy per kilogram necessary to go from point ______________ to point ______________.
-The latent heat of fusion is the amount of energy per kilogram necessary to go from point ______________ to point ______________.
(Short Answer)
4.8/5  (40)
(40)
Which laboratory observation would most support the kinetic theory's assumption that molecules in a gas are widely separated?
(Multiple Choice)
4.9/5  (36)
(36)
A ______________ has a definite volume and assumes the shape of its container.
(Short Answer)
4.8/5  (35)
(35)
The number of molecules in a container is doubled and the Kelvin temperature doubled. The volume remains unchanged. The new pressure will be how many times greater than the original pressure?
(Multiple Choice)
4.7/5  (38)
(38)
At approximately what temperature does water have its greatest density?
(Multiple Choice)
4.9/5  (43)
(43)
The first law of thermodynamics states that heat added to a closed system can change the internal energy of the system and/or do ______________.
(Short Answer)
4.9/5  (32)
(32)
Showing 1 - 20 of 105
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)