Exam 1: Structure and Bonding
Exam 1: Structure and Bonding70 Questions
Exam 2: Acids and Bases48 Questions
Exam 3: Introduction to Organic Molecules and Functional Groups48 Questions
Exam 4: Alkanes56 Questions
Exam 5: Stereochemistry68 Questions
Exam 6: Understanding Organic Reactions43 Questions
Exam 7: Alkyl Halides and Nucleophilic Substitution64 Questions
Exam 8: Alkyl Halides and Elimination Reactions45 Questions
Exam 9: Alcohols, Ethers and Epoxides49 Questions
Exam 10: Alkenes47 Questions
Exam 11: Alkynes45 Questions
Exam 12: Oxidation and Reduction43 Questions
Exam 13: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy43 Questions
Exam 15: Radical Reactions43 Questions
Exam 16: Conjugation, Resonance, and Dienes44 Questions
Exam 17: Benzene and Aromatic Compounds40 Questions
Exam 18: Electrophilic Aromatic Substitution51 Questions
Exam 19: Carboxylic Acids and the Acidity of the O-H Bond44 Questions
Exam 20: Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation and Reduction53 Questions
Exam 21: Aldehydes and Ketones Nucleophilic Addition45 Questions
Exam 22: Carboxylic Acids and Derivatives47 Questions
Exam 23: Substitution Reactions of Carbonyl Compounds at the Alpha-Carbon41 Questions
Exam 24: Carbonyl Condensation Reactions45 Questions
Exam 25: Amines58 Questions
Exam 26: Carbon-Carbon Bond Forming Reactions in Organic Synthesis41 Questions
Exam 27: Pericyclic Reactions52 Questions
Exam 28: Carbohydrates41 Questions
Exam 29: Amino Acids and Proteins41 Questions
Exam 30: Lipids41 Questions
Exam 31: Synthetic Polymers41 Questions
Select questions type
Which of the following statements about valence electrons is true?
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following would you expect to have ionic bonds?
(Multiple Choice)
4.9/5  (41)
(41)
Arrange the following bonds in decreasing order of ionic character, putting the most ionic first. C-C C-N C-O Na-O I II III IV
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following resonance structures is the least important contributor to the resonance hybrid of the formate anion, HCOO-? 
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following molecules has a net dipole moment of zero? 
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following molecules does not have a net dipole moment of zero?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is the appropriate conversion of (CH3)2CHOCH2CH2CH2OH to a skeletal structure? 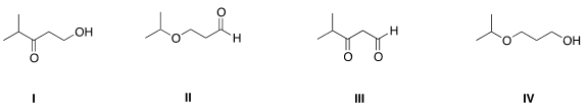
(Multiple Choice)
4.9/5  (33)
(33)
Which is more important in each pair of contributing resonance structures? 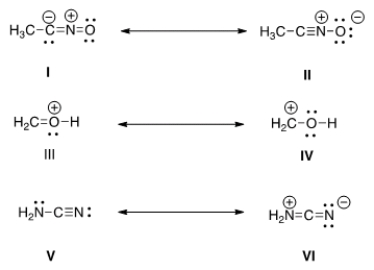
(Multiple Choice)
4.8/5  (35)
(35)
How many constitutional isomers are there for a molecule having the molecular formula C2H6O?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is the appropriate conversion of the condensed structure, CH3COCH3, to a Lewis structure? 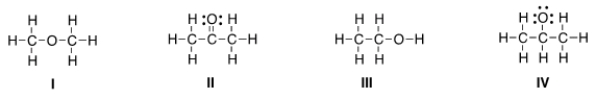
(Multiple Choice)
4.8/5  (38)
(38)
Showing 61 - 70 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)