Exam 37: Molecules
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
The characteristic energy of rotation E0r for the O2 molecule is 1.78 10-4 eV.Calculate the frequency of the photon absorbed for the excitation from the l = 3 to l = 4 rotational level of this diatomic molecule.
(Multiple Choice)
4.7/5  (29)
(29)
Transitions between vibrational states of different electronic states result in the emission of photons in or near the _______ portion of the electromagnetic spectrum.
(Multiple Choice)
4.8/5  (35)
(35)
The energies due to the electronic excitations of a molecule are of the order of 1 eV,whereas
(Multiple Choice)
4.7/5  (37)
(37)
When a NaCl molecule is formed from neutral Na and Cl atoms,
(Multiple Choice)
4.8/5  (28)
(28)
Gases such as CO2 and methane are considered greenhouse gases.This is because
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following statements about covalent bonding is true?
(Multiple Choice)
4.8/5  (27)
(27)
The energy released by an atom's acquisition of one electron is called its
(Multiple Choice)
4.8/5  (34)
(34)
The effective force constant of the CO molecule is 1.86 kN/m.With 12 u for the mass of the carbon atom and 16 u for the mass of the oxygen atom,the frequency of vibration of the CO molecule is
(Multiple Choice)
4.9/5  (31)
(31)
The dissociation energy of an ionic compound is the negative of the potential energy of the positive-negative ion system when the ions are
(Multiple Choice)
4.7/5  (36)
(36)
If the electrostatic potential energy of Na+ and F- ions at their equilibrium separation (with U = 0 at infinite separation)is -1.19 10-18 J,then their equilibrium separation must be approximately
(Multiple Choice)
4.8/5  (34)
(34)
The _______ bond is responsible for the bonding of identical or similar atoms.
(Multiple Choice)
4.7/5  (36)
(36)
The reduced mass of a two-body system in which the masses of the bodies are 2 u and 5 u is
(Multiple Choice)
4.9/5  (41)
(41)
The magnetron of a microwave oven generates a frequency of 2.45 GHz.This frequency corresponds to the first vibrational level, 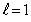 ,of a water molecule.Assuming that the energy is given by
,of a water molecule.Assuming that the energy is given by 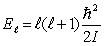 ,the moment of inertia of the water molecule is
,the moment of inertia of the water molecule is
(Multiple Choice)
4.9/5  (40)
(40)
In the absorption spectrum of the diatomic molecule HCl the central frequency is at 8.67 1013 Hz,while the absorption peaks on either side are separated by 5.9 1011 Hz.What is the energy of the lowest vibrational state?
(Multiple Choice)
4.8/5  (37)
(37)
Which one of the following statements is true of the van der Waals bond?
(Multiple Choice)
4.9/5  (39)
(39)
The reduced mass of a two-body system is 2.1 u.If one of the masses is 3 u,then what is the mass of the second body?
(Multiple Choice)
4.7/5  (34)
(34)
In the absorption spectrum of the diatomic molecule HCl the central frequency is at 8.67 1013 Hz,while the absorption peaks on either side are separated by 5.9 1011 Hz.What is the average moment of inertia of a rotating HCl molecule?
(Multiple Choice)
4.8/5  (36)
(36)
If the H2 molecule has a rotational energy of E0r = 0.040 MeV,what is the average distance between the two atoms?
(Multiple Choice)
4.8/5  (33)
(33)
From the frequency of vibration the effective force constant of the HCl molecule is found to be 840 N/m.In order to appreciate the stiffness of the binding force,or imaginary "spring," between the H and Cl,calculate how far a spring of the same stiffness would extend if a 10 kg weight were hung from it.
(Multiple Choice)
4.9/5  (32)
(32)
Showing 21 - 39 of 39
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)