Exam 2: Measurement
Exam 1: Foundations30 Questions
Exam 2: Measurement26 Questions
Exam 3: Atoms30 Questions
Exam 4: Light and Electronic Structure30 Questions
Exam 5: Chemical Bonds Compounds56 Questions
Exam 6: Chemical Reactions47 Questions
Exam 7: Mass Stoichiometry44 Questions
Exam 8: Energy39 Questions
Exam 9: Covalent Bonding and Molecules30 Questions
Exam 10: Solids, Liquids, and Gases30 Questions
Exam 11: Solutions30 Questions
Exam 12: Acids Bases30 Questions
Exam 13: Reaction Rates Equilibrium40 Questions
Exam 14: Oxidation-Reduction Reactions52 Questions
Exam 15: Organic Chemistry and Biomolecules65 Questions
Exam 16: Nuclear Chemistry52 Questions
Select questions type
The distribution of hits on the bull's-eye is described as: 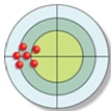
(Multiple Choice)
4.8/5  (42)
(42)
A solution has a mass of 17.41 grams and a volume of 14.4 mL.What is the density of this solution,reported to the correct number of significant digits?
(Multiple Choice)
4.9/5  (41)
(41)
A student measures the volume of a solution to be 0.03010 L.How many significant digits are in this measurement?
(Multiple Choice)
4.8/5  (28)
(28)
Showing 21 - 26 of 26
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)