Exam 9: Covalent Bonding and Molecules
Exam 1: Foundations30 Questions
Exam 2: Measurement26 Questions
Exam 3: Atoms30 Questions
Exam 4: Light and Electronic Structure30 Questions
Exam 5: Chemical Bonds Compounds56 Questions
Exam 6: Chemical Reactions47 Questions
Exam 7: Mass Stoichiometry44 Questions
Exam 8: Energy39 Questions
Exam 9: Covalent Bonding and Molecules30 Questions
Exam 10: Solids, Liquids, and Gases30 Questions
Exam 11: Solutions30 Questions
Exam 12: Acids Bases30 Questions
Exam 13: Reaction Rates Equilibrium40 Questions
Exam 14: Oxidation-Reduction Reactions52 Questions
Exam 15: Organic Chemistry and Biomolecules65 Questions
Exam 16: Nuclear Chemistry52 Questions
Select questions type
Which compound has 24 valence electrons?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
A
Which statement is CORRECT concerning the cyanide ion? 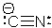
Free
(Multiple Choice)
4.9/5  (27)
(27)
Correct Answer:
C
What is the formal charge on the nitrogen atom in this structure? 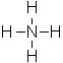
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
A
How many atoms in this molecule have a trigonal planar geometry? 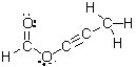
(Multiple Choice)
4.9/5  (39)
(39)
How many atoms in this molecule have a tetrahedral geometry? 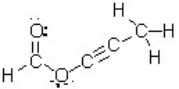
(Multiple Choice)
4.7/5  (32)
(32)
Which molecule does NOT have a lone pair on the central atom?
(Multiple Choice)
5.0/5  (29)
(29)
The Lewis structure of the cyanide ion is shown here.How many valence electrons are in this ion? 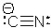
(Multiple Choice)
4.8/5  (31)
(31)
Which compound has an expanded octet around the central atom?
(Multiple Choice)
4.9/5  (39)
(39)
What is the electronic geometry around the oxygen atoms in CO2? 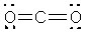
(Multiple Choice)
4.8/5  (32)
(32)
A oxygen atom with one bond and three lone pairs has a charge of:
(Multiple Choice)
4.9/5  (36)
(36)
What is the correct ELECTRONIC GEOMETRY and MOLECULAR GEOMETRY around the central nitrogen in NO2-? (Hint: You may need to draw the molecule.)
(Multiple Choice)
4.8/5  (32)
(32)
Showing 1 - 20 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)