Short Answer
How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 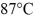 to produce water at a final temperature of
to produce water at a final temperature of 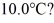 Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: When a fixed amount of ideal gas
Q18: The filament in a light bulb has
Q19: A blacksmith is flattening a steel plate
Q20: A heat conducting rod,1.40 m long,is made
Q21: A cube at 100.0°C radiates heat at
Q23: A heat conducting rod,0.90 m long,is made
Q24: The figure shows the pV diagram for
Q26: A spherical object 25.0 cm in diameter
Q27: An expansion process on an ideal diatomic
Q51: When a fixed amount of ideal gas