Short Answer
The figure shows the pV diagram for a certain thermodynamic process.In this process,
1500 J of heat flows into a system,and at the same time the system expands against a constant external pressure of 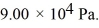 If the volume of the system increases from
If the volume of the system increases from 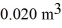 to
to 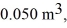 calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative.
calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative. 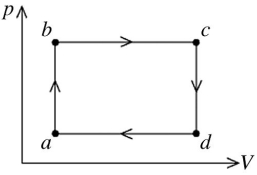
Correct Answer:

Verified
Correct Answer:
Verified
Q2: When a fixed amount of ideal gas
Q19: A blacksmith is flattening a steel plate
Q20: A heat conducting rod,1.40 m long,is made
Q22: How many grams of ice at -13°C
Q23: A heat conducting rod,0.90 m long,is made
Q26: A spherical object 25.0 cm in diameter
Q27: An expansion process on an ideal diatomic
Q28: The temperature of an ideal gas in
Q29: A container with rigid walls is filled
Q51: When a fixed amount of ideal gas