Multiple Choice
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 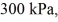 the initial volume is
the initial volume is 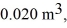 and the initial temperature is
and the initial temperature is 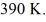 The final pressure is
The final pressure is 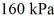 and the final temperature is
and the final temperature is 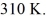 The change in the internal (thermal) energy of the gas is closest to
The change in the internal (thermal) energy of the gas is closest to
A) -3100 J.
B) -1800 J.
C) 3100 J.
D) 1800 J.
E) 0.00 J.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: When a fixed amount of ideal gas
Q22: How many grams of ice at -13°C
Q23: A heat conducting rod,0.90 m long,is made
Q24: The figure shows the pV diagram for
Q26: A spherical object 25.0 cm in diameter
Q28: The temperature of an ideal gas in
Q29: A container with rigid walls is filled
Q34: An ideal gas is compressed in a
Q37: A cylindrical bar that us well insulated
Q39: It is a well-known fact that water