Multiple Choice
The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container.The temperature T1 of the gas in state 1 is 21°C.What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K,and the ATOMIC weight of oxygen is 16 g/mol. 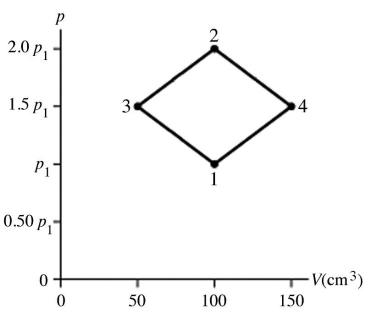
A) -52°C,390°C
B) 16°C,47°C
C) 220°C,660°C
D) 11°C,32°C
Correct Answer:

Verified
Correct Answer:
Verified
Q7: During an adiabatic process, 20 moles of
Q14: A 905-g meteor impacts the earth at
Q31: A certain amount of ideal monatomic gas
Q40: An ideal gas in a balloon is
Q41: A quantity of ideal gas requires 800
Q42: A 648-g empty iron kettle is put
Q46: A steel container,equipped with a piston,contains 21
Q47: A fixed amount of ideal gas goes
Q48: An expansion process on an ideal diatomic
Q49: The gas in a perfectly insulated system