Multiple Choice
The temperature of an ideal gas in a sealed 0.40- 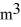 rigid container is reduced from 350 K to
rigid container is reduced from 350 K to 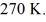 The final pressure of the gas is
The final pressure of the gas is 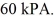 The molar heat capacity at constant volume of the gas is 28.0 J/mol · K.The heat absorbed by the gas is closest to
The molar heat capacity at constant volume of the gas is 28.0 J/mol · K.The heat absorbed by the gas is closest to
A) -24 kJ.
B) -31 kJ.
C) 24 kJ.
D) 31 kJ.
E) 0.00 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: When a fixed amount of ideal gas
Q23: A heat conducting rod,0.90 m long,is made
Q24: The figure shows the pV diagram for
Q26: A spherical object 25.0 cm in diameter
Q27: An expansion process on an ideal diatomic
Q29: A container with rigid walls is filled
Q34: An ideal gas is compressed in a
Q36: In an isochoric process,the internal (thermal)energy of
Q37: A cylindrical bar that us well insulated
Q39: It is a well-known fact that water