Multiple Choice
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 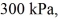 the initial volume is
the initial volume is 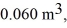 and the initial temperature is
and the initial temperature is 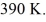 The ideal gas constant is R = 8.314 J/mol ∙ K.The final pressure is
The ideal gas constant is R = 8.314 J/mol ∙ K.The final pressure is 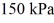 and the final temperature is
and the final temperature is 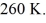 The work done by the gas is closest to
The work done by the gas is closest to
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: During an adiabatic process, 20 moles of
Q11: A 400-g piece of metal at 120.0°C
Q14: A 905-g meteor impacts the earth at
Q31: A certain amount of ideal monatomic gas
Q44: The figure shows a pV diagram for
Q47: A fixed amount of ideal gas goes
Q49: The gas in a perfectly insulated system
Q49: In an isochoric process,the internal (thermal)energy of
Q52: Heat is added to a pure substance
Q53: What is the steady state rate of