Essay
A fixed amount of ideal gas goes through a process abc.In state a,the temperature of the gas is 152°C,its pressure is 1.25 atm,and it occupies a volume of 0.250 m3.It then undergoes an isothermal expansion to state b that doubles its volume,followed by an isobaric compression back to its original volume at state c.(Hint: First show this process on a pV diagram.)The ideal gas constant is 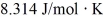 ,and 1.00 atm = 1.01 × 105 Pa.
,and 1.00 atm = 1.01 × 105 Pa.
(a)How many moles does this gas contain?
(b)What is the change in the internal energy of the gas between states a and b?
(c)What is the net work done on (or by)this gas during the entire process?
(d)What is the temperature of the gas in state c?
Correct Answer:

Verified
(a)8.93 mo...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q7: During an adiabatic process, 20 moles of
Q11: A 400-g piece of metal at 120.0°C
Q14: A 905-g meteor impacts the earth at
Q31: A certain amount of ideal monatomic gas
Q42: A 648-g empty iron kettle is put
Q44: The figure shows a pV diagram for
Q48: An expansion process on an ideal diatomic
Q49: The gas in a perfectly insulated system
Q49: In an isochoric process,the internal (thermal)energy of
Q52: Heat is added to a pure substance