Multiple Choice
Write a balanced half-reaction for the reduction of CrO42-(aq) to Cr(OH) 3(s) in a basic solution.
A) CrO42-(aq) + 3 OH-(aq) + 3 e- → Cr(OH) 3(s) + 2 O2(g)
B) CrO42-(aq) + 3 H+(aq) + 3 e- → Cr(OH) 3(s)
C) CrO42-(aq) + 3 H+(aq) → Cr(OH) 3(s) + 2 e-
D) CrO42-(aq) + 4 H2O( 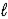 ) + 3 e- → Cr(OH) 3(s) + 5 OH-(aq)
) + 3 e- → Cr(OH) 3(s) + 5 OH-(aq)
E) CrO42-(aq) + 3 OH-(aq) → Cr(OH) 3(s) + 2 O2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Which of the following statements is true
Q17: Which of the following are the expected
Q18: The unit for electromotive force,emf,is the volt.1
Q19: Which of the following is the cell
Q20: Write a balanced net ionic equation for
Q22: Calculate the equilibrium constant for the following
Q23: Calculate the standard cell potential ( <img
Q24: Primary batteries are also called storage batteries
Q25: For the following cell reaction,the standard cell
Q26: Which of the following is the cell