Multiple Choice
Calculate the equilibrium constant for the following reaction at 25 °C, 2 IO3-(aq) + 5 Hg( 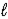 ) + 12 H+(aq) → I2(s) + 5 Hg2+(aq) + 6 H2O(
) + 12 H+(aq) → I2(s) + 5 Hg2+(aq) + 6 H2O( 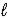 )
)
The standard reduction potentials are as follows:
IO3-(aq) + 6 H+(aq) + 5 e- → I2(s) + 3 H2O( 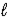 )
)
E° = +1.20 V
Hg2+(aq) + 2 e- → Hg( 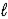 )
)
E° = +0.86 V
A) 3 × 10-58
B) 6 × 105
C) 3 × 1011
D) 6 × 1028
E) 3 × 1057
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Which of the following are the expected
Q18: The unit for electromotive force,emf,is the volt.1
Q19: Which of the following is the cell
Q20: Write a balanced net ionic equation for
Q21: Write a balanced half-reaction for the reduction
Q23: Calculate the standard cell potential ( <img
Q24: Primary batteries are also called storage batteries
Q25: For the following cell reaction,the standard cell
Q26: Which of the following is the cell
Q27: Which of the following reactions will require