Multiple Choice
Use the following standard reduction potentials to determine which species is the best oxidizing agent. O2(g) + 4 H+(aq) + 4 e- → 2 H2O( 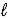 )
)
E° = +1.229 V
Hg22+(aq) + 2 e- → 2 Hg( 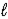 )
)
E° = +0.789 V
I2(s) + 2 e- → 2 I-(aq)
E° = +0.535 V
A) I2(s)
B) O2(g)
C) I-(aq)
D) Hg22+(aq)
E) H2O( 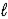 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Consider the following half-reactions. Ag<sup>+</sup>(aq)+ e<sup>-</sup> →
Q67: Which of the following statements is true
Q68: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="Calculate for
Q69: Write a balanced net ionic equation for
Q70: In the given electrochemical cell,which of the
Q72: A voltaic cell or galvanic cell converts
Q73: How many electrons are transferred in the
Q74: Write a balanced net ionic equation for
Q75: Use the following standard reduction potentials to
Q76: Write a balanced chemical equation for the