Multiple Choice
Which of the following statements is true concerning the voltaic cell shown below? 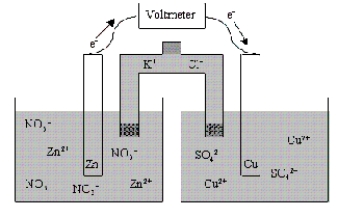
A) The Zn anode mass decreases as the cell discharges.
B) The Zn cathode mass increases as the cell discharges.
C) The Zn cathode mass decreases as the cell discharges.
D) The Zn anode mass increases as the cell discharges.
E) The mass of the Zn electrode neither increases nor decreases as the cell discharges.
Correct Answer:

Verified
Correct Answer:
Verified
Q62: If Δ<sub>r</sub>G° for the following reaction is
Q63: How many moles of electrons are produced
Q64: Calculate the equilibrium constant for the reaction
Q65: If an electric current is passed through
Q66: Consider the following half-reactions. Ag<sup>+</sup>(aq)+ e<sup>-</sup> →
Q68: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="Calculate for
Q69: Write a balanced net ionic equation for
Q70: In the given electrochemical cell,which of the
Q71: Use the following standard reduction potentials to
Q72: A voltaic cell or galvanic cell converts