Multiple Choice
Calculate 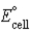 for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
Pb2+(aq) + 2 e- → Pb(s)
E° = -0.126 V
PbCl2(s) + 2 e- → Pb(s) + 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
Fe2+(aq) + e- → Fe(s)
E° = -0.44 V
A) -0.504 V
B) -0.062 V
C) +0.504 V
D) +1.038 V
E) +1.604 V
Correct Answer:

Verified
Correct Answer:
Verified
Q63: How many moles of electrons are produced
Q64: Calculate the equilibrium constant for the reaction
Q65: If an electric current is passed through
Q66: Consider the following half-reactions. Ag<sup>+</sup>(aq)+ e<sup>-</sup> →
Q67: Which of the following statements is true
Q69: Write a balanced net ionic equation for
Q70: In the given electrochemical cell,which of the
Q71: Use the following standard reduction potentials to
Q72: A voltaic cell or galvanic cell converts
Q73: How many electrons are transferred in the