Multiple Choice
Write a balanced chemical equation which corresponds to the following equilibrium constant expression. 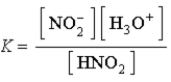
A) HNO2(aq) + H2O( 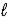 )
)  NO2-(aq) + H3O+(aq)
NO2-(aq) + H3O+(aq)
B) NO2-(aq) + H3O+(aq) 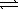 HNO2(aq) + H2O(
HNO2(aq) + H2O( 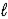 )
)
C) NO2-(aq) + H3O+(aq) 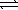 HNO2(aq)
HNO2(aq)
D) H+(aq) + OH-(aq) 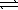 H2O(
H2O( 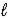 )
)
E) HNO2(aq) 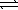 NO2-(aq) + H3O+(aq)
NO2-(aq) + H3O+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Consider the reaction A(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="Consider
Q15: The equilibrium constant (K<sub>c</sub>)for the decomposition of
Q16: In 1913,the Haber-Bosch process was patented.The product
Q17: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="For
Q18: At a given temperature,K = 0.024 for
Q20: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is placed
Q21: Which of the following expressions for K
Q22: The symbol Q is called the _.
Q23: When 0.20 mole HF is dissolved in
Q24: A 3.50-mol sample of HI is placed