Multiple Choice
A 3.50-mol sample of HI is placed in a 1.00-L vessel at 460°C,and the reaction system is allowed to come to equilibrium.The HI partially decomposes,forming 0.266 mol H2 and 0.266 mol I2 at equilibrium.What is the equilibrium constant Kc for the following reaction at 460°C? ½ H2(g) + ½ I2(g) 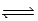 HI(g)
HI(g)
A) 1.23 × 102
B) 8.10 × 10-3
C) 2.40 × 10-2
D) 11.1
E) 6.45
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Write a balanced chemical equation which corresponds
Q20: At a given temperature,0.0664 mol N<sub>2</sub>O<sub>4</sub>(g)is placed
Q21: Which of the following expressions for K
Q22: The symbol Q is called the _.
Q23: When 0.20 mole HF is dissolved in
Q25: The equilibrium constant,K,is always the same within
Q26: A 10.0 g sample of solid NH<sub>4</sub>Cl
Q27: The thermochemical equation for the formation of
Q28: Consider the following equilibrium: C<sub>2</sub>H<sub>6</sub>(g)+ C<sub>5</sub>H<sub>12</sub>(g) <img
Q29: A mixture of nitrogen and hydrogen was