Multiple Choice
Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: 2NF3(g) 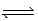 N2(g) + 3F2(g)
N2(g) + 3F2(g)
2) 50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0194 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L⋅atm/mol⋅K)
A) 2.68 × 10-6
B) 1.91 × 10-3
C) 1.79 × 10-3
D) 2.77 × 10-6
E) 4.43 × 10-7
Correct Answer:

Verified
Correct Answer:
Verified
Q25: The equilibrium constant,K,is always the same within
Q26: A 10.0 g sample of solid NH<sub>4</sub>Cl
Q27: The thermochemical equation for the formation of
Q28: Consider the following equilibrium: C<sub>2</sub>H<sub>6</sub>(g)+ C<sub>5</sub>H<sub>12</sub>(g) <img
Q29: A mixture of nitrogen and hydrogen was
Q31: When the pressure of an equilibrium mixture
Q32: If K<sub>c</sub> = 0.124 for A<sub>2</sub> +
Q33: Which of the following is the correct
Q34: A 3.00-liter flask initially contains 3.00 mol
Q35: At a high temperature,equal concentrations of 0.160