Multiple Choice
If Kc = 0.124 for A2 + 2B 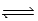 2AB,what is the value of Kc for the reaction 4AB
2AB,what is the value of Kc for the reaction 4AB 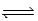 2A2 + 4B?
2A2 + 4B?
A) 0.124
B) 0.248
C) 65.0
D) -0.124
E) 4.03
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: The thermochemical equation for the formation of
Q28: Consider the following equilibrium: C<sub>2</sub>H<sub>6</sub>(g)+ C<sub>5</sub>H<sub>12</sub>(g) <img
Q29: A mixture of nitrogen and hydrogen was
Q30: Nitrogen trifluoride decomposes at to form nitrogen
Q31: When the pressure of an equilibrium mixture
Q33: Which of the following is the correct
Q34: A 3.00-liter flask initially contains 3.00 mol
Q35: At a high temperature,equal concentrations of 0.160
Q36: For which of the following reactions is
Q37: Given the equilibrium constants for the equilibria,