Multiple Choice
The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows. N2(g) + 3 H2(g) 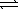 2 NH3(g)
2 NH3(g)
ΔH = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
A) adding N2(g)
B) removing NH3(g)
C) adding a catalyst
D) decreasing the temperature
E) removing H2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The symbol Q is called the _.
Q23: When 0.20 mole HF is dissolved in
Q24: A 3.50-mol sample of HI is placed
Q25: The equilibrium constant,K,is always the same within
Q26: A 10.0 g sample of solid NH<sub>4</sub>Cl
Q28: Consider the following equilibrium: C<sub>2</sub>H<sub>6</sub>(g)+ C<sub>5</sub>H<sub>12</sub>(g) <img
Q29: A mixture of nitrogen and hydrogen was
Q30: Nitrogen trifluoride decomposes at to form nitrogen
Q31: When the pressure of an equilibrium mixture
Q32: If K<sub>c</sub> = 0.124 for A<sub>2</sub> +