Multiple Choice
Nickel crystallizes in a face-centered cubic lattice.The density of the nickel is 8.91 g/cm3.What is the volume of a single unit cell?
A) 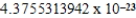 cm3
cm3
B) 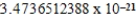 cm3
cm3
C) 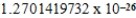 cm3
cm3
D) 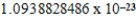 cm3
cm3
E) 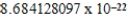 cm3
cm3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: Polonium (atomic mass 209.0 g/mol)crystallizes in a
Q53: What is the length of the diagonal
Q54: For a metal that crystallizes in a
Q55: Cesium bromide crystallizes in a primitive cubic
Q56: Gold (atomic mass 197 g/mol),with an atomic
Q57: Elements that have their highest energy electrons
Q58: Strontium oxide has a face centered cubic
Q59: Which process requires the greatest endothermic change
Q60: Iron crystallizes in a body-centered cubic lattice.If
Q61: Rubidium iodide (molar mass 212.4 g/mol)has a