Multiple Choice
How many sigma and pi bonds are in the molecule pictured below? 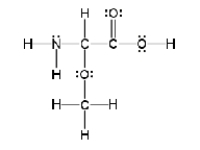
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds
Correct Answer:

Verified
Correct Answer:
Verified
Q1: What is the hybridization of the central
Q27: Refer to Diagram 9-1.Assume that the molecular
Q30: How many sigma (σ)bonds and pi (π)bonds
Q35: Refer to diagram 9-1.Identify the molecule with
Q41: What is the hybridization of the sulfur
Q43: Refer to Diagram 9-1.What is the molecular
Q51: In HF<sub>2</sub>− the hydrogen is shared between
Q53: Refer to Diagram 9-1.According to molecular orbital
Q57: Which molecule will have the following valence
Q60: How many sigma (σ) bonds and pi