Multiple Choice
The reversible reaction: PCl3(g) + Cl2(g)  PCl5(g) ,has K = 0.5. Based on the molecular art shown below,what can be inferred about the reaction conditions?
PCl5(g) ,has K = 0.5. Based on the molecular art shown below,what can be inferred about the reaction conditions? 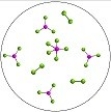
A) The reaction has not yet reached equilibrium.
B) The reaction mixture is at equilibrium.
C) The reaction will shift to the left to reach equilibrium.
D) The forward reaction is favorable.
Correct Answer:

Verified
Correct Answer:
Verified
Q76: In the energy diagram shown below,C labels
Q77: Ammonia (NH<sub>3</sub>)is synthesized by the reaction of
Q78: Which of the following energy quantities is
Q79: An equilibrium constant with a value of
Q80: Consider the reversible reaction: CO(g)+ Cl<sub>2</sub>(g) <img
Q82: When the pressure of a reaction at
Q83: The equilibrium constant expression for the reversible
Q84: When the equilibrium constant for a reaction
Q85: Changes in potential energy occur in chemical
Q86: One step in the metabolism of glucose