True/False
Consider the reversible reaction: CO(g)+ Cl2(g) 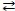 COCl2(g). The reverse reaction is COCl2(g)→ CO(g)+ Cl2(g).
COCl2(g). The reverse reaction is COCl2(g)→ CO(g)+ Cl2(g).
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: A catalytic converter uses a catalyst to
Q76: In the energy diagram shown below,C labels
Q77: Ammonia (NH<sub>3</sub>)is synthesized by the reaction of
Q78: Which of the following energy quantities is
Q79: An equilibrium constant with a value of
Q81: The reversible reaction: PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7327/.jpg"
Q82: When the pressure of a reaction at
Q83: The equilibrium constant expression for the reversible
Q84: When the equilibrium constant for a reaction
Q85: Changes in potential energy occur in chemical