True/False
Ammonia (NH3)is synthesized by the reaction of nitrogen and hydrogen in the presence of an iron catalyst according to the equation below. Removing the iron catalyst would decrease the reaction rate. 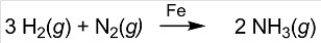
Correct Answer:

Verified
Correct Answer:
Verified
Q72: Consider the reaction: 2 C<sub>2</sub>H<sub>6</sub>(g)+ 7 O<sub>2</sub>(g)→
Q73: Which value (if any)in each pair corresponds
Q74: Consider the following reversible reaction at equilibrium:
Q75: A catalytic converter uses a catalyst to
Q76: In the energy diagram shown below,C labels
Q78: Which of the following energy quantities is
Q79: An equilibrium constant with a value of
Q80: Consider the reversible reaction: CO(g)+ Cl<sub>2</sub>(g) <img
Q81: The reversible reaction: PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7327/.jpg"
Q82: When the pressure of a reaction at