Multiple Choice
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. 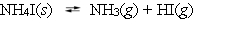
At 400 C, Kp = 0.215. Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400 C.
A) 0.103 atm
B) 0.215 atm
C) 0.232 atm
D) 0.464 atm
E) 2.00 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q48: Consider the equilibrium reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q49: The following reaction is at equilibrium in
Q50: Write the mass-action expression, Q<sub>c</sub>, for the
Q51: The following reaction is at equilibrium at
Q53: The reaction of nitric oxide to
Q54: A mixture of 0.500 mole of carbon
Q55: The equilibrium constant, K<sub>p</sub>, for the
Q56: Consider the reversible reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q57: Carbon monoxide and chlorine combine in an
Q88: In a chemical reaction, if the starting