Multiple Choice
The following reaction is at equilibrium at one atmosphere, in a closed container. 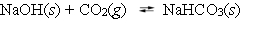 Which, if any, of the following actions will decrease the total amount of CO2 gas present at equilibrium?
Which, if any, of the following actions will decrease the total amount of CO2 gas present at equilibrium?
A) adding N2 gas to double the pressure
B) adding more solid NaOH
C) decreasing the volume of the container
D) removing half of the solid NaHCO3
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q46: At 25 <span class="ql-formula" data-value="\degree"><span
Q48: Consider the equilibrium reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q49: The following reaction is at equilibrium in
Q50: Write the mass-action expression, Q<sub>c</sub>, for the
Q52: Ammonium iodide dissociates reversibly to ammonia
Q53: The reaction of nitric oxide to
Q54: A mixture of 0.500 mole of carbon
Q55: The equilibrium constant, K<sub>p</sub>, for the
Q56: Consider the reversible reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q88: In a chemical reaction, if the starting