Multiple Choice
A mixture of 0.500 mole of carbon monoxide and 0.400 mole of bromine was placed into a rigid 1.00-L container and the system was allowed to come to equilibrium. The equilibrium concentration of COBr2 was 0.233 M. What is the value of Kc for this reaction? 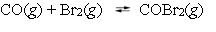
A) 5.23
B) 1.22
C) 1.165
D) 0.858
E) 0.191
Correct Answer:

Verified
Correct Answer:
Verified
Q49: The following reaction is at equilibrium in
Q50: Write the mass-action expression, Q<sub>c</sub>, for the
Q51: The following reaction is at equilibrium at
Q52: Ammonium iodide dissociates reversibly to ammonia
Q53: The reaction of nitric oxide to
Q55: The equilibrium constant, K<sub>p</sub>, for the
Q56: Consider the reversible reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q57: Carbon monoxide and chlorine combine in an
Q58: Nitrogen dioxide decomposes according to the
Q59: At high temperatures, carbon reacts with O<sub>2</sub>