Multiple Choice
Magnesium carbonate dissociates to magnesium oxide and carbon dioxide at elevated temperatures. 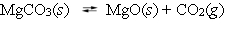 A reaction vessel contains these compounds in equilibrium at 300 C. What will happen if the volume of the container is reduced by 25% at 300 C?
A reaction vessel contains these compounds in equilibrium at 300 C. What will happen if the volume of the container is reduced by 25% at 300 C?
A) The partial pressure of carbon dioxide present at equilibrium will increase.
B) The partial pressure of carbon dioxide present at equilibrium will decrease.
C) The partial pressure of carbon dioxide at equilibrium will be unchanged.
D) The equilibrium constant will have to decrease to compensate for the decrease in volume.
E) More information is needed in order to make a valid judgment.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Write the mass-action expression, Q<sub>c</sub>, for the
Q32: The reaction of nitrogen with oxygen
Q33: The following reaction is at equilibrium at
Q36: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction
Q37: A mixture of 0.600 mol of
Q38: Write the expressions for K<sub>c</sub> and K<sub>p</sub>
Q39: The following reaction is at equilibrium in
Q40: Ethane can be formed by reacting acetylene
Q53: When a reaction system reaches equilibrium, the
Q78: If all the reactants and products in