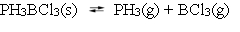Related Questions
Q33: The following reaction is at equilibrium at
Q35: Magnesium carbonate dissociates to magnesium oxide
Q36: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction
Q37: A mixture of 0.600 mol of
Q39: The following reaction is at equilibrium in
Q40: Ethane can be formed by reacting acetylene
Q41: Write the mass-action expression, Q<sub>c</sub> , for
Q42: Sodium hydrogen carbonate decomposes above 110
Q43: At a certain temperature the reaction CO<sub>2</sub>(g)
Q53: When a reaction system reaches equilibrium, the