Multiple Choice
Based on the initial rate data below, what is the value of the rate constant? 2NOBr(g) 2NO(g) + Br2(g) 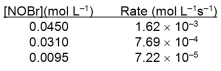
A) 0.0360 L mol¯1s¯1
B) 0.800 L mol¯1s¯1
C) 1.25 L mol¯1s¯1
D) 27.8 L mol¯1s¯1
E) 0.0360 s¯1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: The rate of a reaction is determined
Q71: The rate constant for a reaction is
Q80: For the reaction 3A(g) + 2B(g)
Q81: In the gas phase at 500.
Q83: In the collision theory of reaction rates,
Q84: Consider the following mechanism for the
Q85: Carbon-14 is a radioactive isotope which decays
Q86: The decomposition of dinitrogen pentaoxide to
Q88: The rate constant for the reaction
Q89: Consider this reaction: 8A(g) + 5B(g)