Multiple Choice
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. 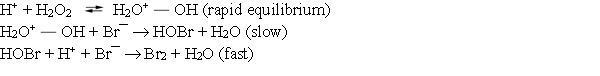 What is the overall reaction equation for this process?
What is the overall reaction equation for this process?
A) 2H2O+  OH + 2Br¯ H2O2 + Br2 + 2H2O
OH + 2Br¯ H2O2 + Br2 + 2H2O
B) 2H+ + 2Br¯ + H2O2 Br2 + 2H2O
C) 2H+ + H2O2 + Br¯ + HOBr H2O+  OH + Br2 + H2O
OH + Br2 + H2O
D) H2O+  OH + Br¯ + H+ Br2 + H2O
OH + Br¯ + H+ Br2 + H2O
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: The rate of a reaction is determined
Q71: The rate constant for a reaction is
Q80: For the reaction 3A(g) + 2B(g)
Q81: In the gas phase at 500.
Q83: In the collision theory of reaction rates,
Q85: Carbon-14 is a radioactive isotope which decays
Q86: The decomposition of dinitrogen pentaoxide to
Q87: Based on the initial rate data
Q88: The rate constant for the reaction
Q89: Consider this reaction: 8A(g) + 5B(g)