Multiple Choice
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
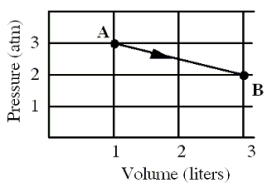
-Suppose that the same gas is originally in state A as described above,but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
A) The change in the internal energy is zero.
B) The final state of the system will still be B.
C) The work done will be smaller for the isothermal process.
D) The heat added will be smaller for the isothermal process.
E) The heat added for the isothermal process will be equal to the work done.
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Complete the following statement: The first law
Q36: Beneath the moveable piston in the drawing,2.25
Q37: Which one of the following pressure-volume graphs
Q38: An ideal monatomic gas undergoes an adiabatic
Q39: A thermally isolated sample of an ideal
Q41: A system containing an ideal gas at
Q42: A heat engine operates between a hot
Q43: What are the SI units of the
Q44: A match ignites within in an oxygen-filled
Q45: Which one of the following statements is