Multiple Choice
An ideal monatomic gas expands isobarically from state A to state B. It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.
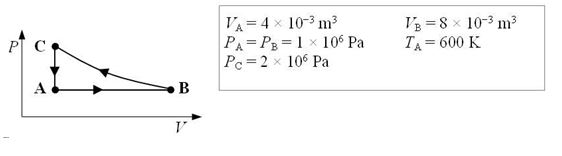
-How much work is done on the gas in going from B to C?
A) 2.5 × 106 J
B) 5.5 × 106 J
C) 4.5 × 106 J
D) 6.5 × 106 J
E) 8.0 × 106 J
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Enclosed beneath the moveable piston in the
Q17: A quantity of carbon monoxide gas is
Q18: An ideal monatomic gas expands isobarically from
Q20: A container is divided into two chambers
Q22: 5.00 kg of liquid water is heated
Q25: 5.00 kg of liquid water is heated
Q29: An ideal monatomic gas expands isothermally from
Q40: An ideal monatomic gas originally in state
Q48: An ideal monatomic gas expands isothermally from
Q53: Which one of the following statements best