Multiple Choice
Enclosed beneath the moveable piston in the drawing is 4.8 moles of a monatomic ideal gas. The gas performs work on the piston as 2300 J of heat are added from the surroundings. During the process, the temperature of the gas decreases by 45 K. How much work does the gas perform? 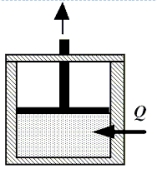
A) 5.0 × 103 J
B) 3.2 × 103 J
C) 1.4 × 103 J
D) 6.0 × 102 J
E) 4.4 × 103 J
Correct Answer:

Verified
Correct Answer:
Verified
Q12: In a reversible heat engine, one mole
Q13: Two moles of an ideal gas have
Q14: A thermally isolated sample of an ideal
Q17: A quantity of carbon monoxide gas is
Q18: An ideal monatomic gas expands isobarically from
Q20: A Carnot engine operates between hot and
Q20: A container is divided into two chambers
Q21: An ideal monatomic gas expands isobarically from
Q53: Which one of the following statements best
Q77: An ideal monatomic gas expands isobarically from