Multiple Choice
Phosphoryl iodide is used in the preparation of organophosphorus derivatives and phosphate esters. Select the Lewis structure for POI3 that minimizes formal charges.
A) 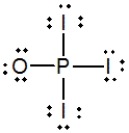
B) 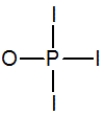
C) 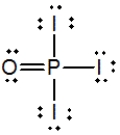
D) 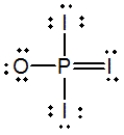
E) 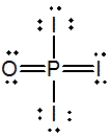
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which one of the following molecules has
Q3: In the following Lewis structure for ClO<sub>3</sub>F,
Q4: Select the Lewis structure for XeO<sub>2</sub>F<sub>2 </sub>that
Q5: The Lewis structure of NO<sub>2</sub> violates the
Q6: All possible resonance structures contribute equally to
Q7: Boron never achieves an octet in any
Q8: Select the best Lewis structure for P<sub>2</sub>I<sub>4</sub>.<br>A)
Q9: Which one of the following molecules contains
Q10: Predict the ideal bond angles in GeCl<sub>4
Q11: When resonance occurs, the bond lengths in