Multiple Choice
In the subshell of the Li2+ ion with orbital quantum number 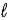 , the allowed values of the magnetic quantum number
, the allowed values of the magnetic quantum number 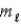 are
are
A) − 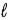 to
to 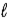
B) −(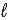 + 1) to(
+ 1) to( 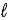 + l)
+ l)
C) − ( 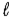 + 2) to (
+ 2) to (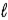 + 2)
+ 2)
D) −(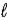 + 3) to (
+ 3) to (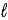 + 3)
+ 3)
E) 0 to n − 1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: In the Bohr model of the hydrogen
Q29: If P(r) is the radial probability density
Q30: Light is emitted by hydrogen atoms in
Q31: The number of states in the He<sup>+</sup>
Q32: The radial portion of the de Broglie
Q34: A hydrogen atom emits a photon of
Q35: An electron in a hydrogen atom makes
Q36: In a completely filled atomic shell,<br>A) the
Q37: How fast is the electron moving in
Q38: Characteristic x-rays can be produced by bombarding