Multiple Choice
The radial portion of the de Broglie wavefunction for an electron in the ground state of the hydrogen atom is Ψ1s(r) = 1/( 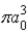 ) 1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
) 1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
A) 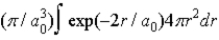
B) 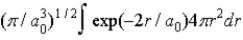
C) 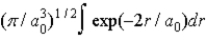
D) 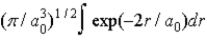
E) 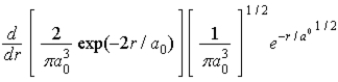
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: A Li<sup>2+</sup> ion undergoes a transition from
Q28: In the Bohr model of the hydrogen
Q29: If P(r) is the radial probability density
Q30: Light is emitted by hydrogen atoms in
Q31: The number of states in the He<sup>+</sup>
Q33: In the subshell of the Li<sup>2+</sup> ion
Q34: A hydrogen atom emits a photon of
Q35: An electron in a hydrogen atom makes
Q36: In a completely filled atomic shell,<br>A) the
Q37: How fast is the electron moving in