Multiple Choice
If P(r) is the radial probability density function for an electron in the ground state of a hydrogen atom, the most probable value for r can be found from
A) dP/dt
B) dP/dr
C) 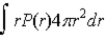
D) 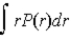
E) d2P/dr2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: One of the main problems with the
Q25: Adam and Eve are contemplating the beauty
Q26: The energy difference between the upper and
Q27: A Li<sup>2+</sup> ion undergoes a transition from
Q28: In the Bohr model of the hydrogen
Q30: Light is emitted by hydrogen atoms in
Q31: The number of states in the He<sup>+</sup>
Q32: The radial portion of the de Broglie
Q33: In the subshell of the Li<sup>2+</sup> ion
Q34: A hydrogen atom emits a photon of