Multiple Choice
The s, p, d, f, symbols represent values of the quantum number
A) ms
B) n
C) 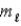
D) 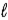
E) mj
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: What angle does the orbital angular momentum
Q18: A hydrogen atom in the 4f state
Q19: Forbidden transitions and selection rules suggest that<br>A)
Q20: In a shell of the hydrogen atom
Q21: Quantum physics agrees with the classical physics
Q23: Which of the following statements is true?<br>A)<br><img
Q24: One of the main problems with the
Q25: Adam and Eve are contemplating the beauty
Q26: The energy difference between the upper and
Q27: A Li<sup>2+</sup> ion undergoes a transition from