Multiple Choice
Adam and Eve are contemplating the beauty of the hydrogen atom. Adam claims that the quantum states with a given value of the principal quantum number n can have any value of the orbital quantum number 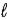 . Eve says that the Snake told her that a state with a given value of
. Eve says that the Snake told her that a state with a given value of 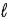 could have any value of n. Which one, if either, is correct, and why?
could have any value of n. Which one, if either, is correct, and why?
A) Adam, because the man is always right.
B) Adam because n ≤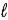 − 1.
− 1.
C) Eve, because n ≤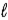 − 1.
− 1.
D) Eve, because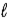 ≤ n − 1.
≤ n − 1.
E) Neither, because Adam is wrong and the Snake told a subtle lie.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: In a shell of the hydrogen atom
Q21: Quantum physics agrees with the classical physics
Q22: The s, p, d, f, symbols represent
Q23: Which of the following statements is true?<br>A)<br><img
Q24: One of the main problems with the
Q26: The energy difference between the upper and
Q27: A Li<sup>2+</sup> ion undergoes a transition from
Q28: In the Bohr model of the hydrogen
Q29: If P(r) is the radial probability density
Q30: Light is emitted by hydrogen atoms in