Multiple Choice
One of the main problems with the Bohr model of the hydrogen atom when compared with the results of the methods of quantum mechanics used to describe atoms, was that the Bohr model predicted
A) the ground state angular momentum was L = 1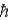 .
.
B) the frequency of the radiation emitted when an electron "jumps" from one allowed orbit to another was hf = Ei − Ef.
C) the potential energy function for the hydrogen atom was given by V(r) = −ke2/r.
D) the energy of the ground state of the hydrogen atom was En = −13.6 eV.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Forbidden transitions and selection rules suggest that<br>A)
Q20: In a shell of the hydrogen atom
Q21: Quantum physics agrees with the classical physics
Q22: The s, p, d, f, symbols represent
Q23: Which of the following statements is true?<br>A)<br><img
Q25: Adam and Eve are contemplating the beauty
Q26: The energy difference between the upper and
Q27: A Li<sup>2+</sup> ion undergoes a transition from
Q28: In the Bohr model of the hydrogen
Q29: If P(r) is the radial probability density