Multiple Choice
If the exothermic reaction 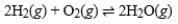 has reached equilibrium, what is the effect of reducing the temperature of the reaction vessel?
has reached equilibrium, what is the effect of reducing the temperature of the reaction vessel?
A) The reaction will shift from the left to the right.
B) The reaction will shift from the right to the left.
C) There will be no effect.
D) Whether the reaction will shift to the left or the right depends on the initial temperature.
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Consider the following graph. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="Consider
Q30: The term "heterogeneous catalyst" means which of
Q31: How does the rate of reaction change
Q32: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For the
Q33: Suppose the equilibrium constant for the chemical
Q35: For the exothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For
Q36: Which of the following statements is true
Q37: Which of the following is true of
Q38: Consider the following graph. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="Consider
Q39: A particular reaction has an equilibrium constant