Multiple Choice
Consider the following graph. 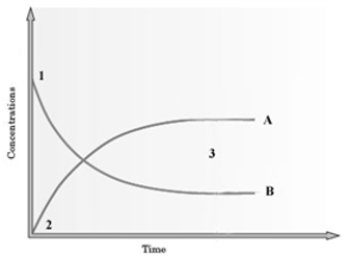 The graph is based on data collected from the following reaction.
The graph is based on data collected from the following reaction. 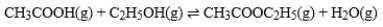 At what point on the graph does the rate of the forward reaction equal the rate of the reverse reaction?
At what point on the graph does the rate of the forward reaction equal the rate of the reverse reaction?
A) 1
B) 2
C) 3
D) The point of intersection of A and B
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Suppose the equilibrium constant for the chemical
Q34: If the exothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="If
Q35: For the exothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For
Q36: Which of the following statements is true
Q37: Which of the following is true of
Q39: A particular reaction has an equilibrium constant
Q40: What is the purpose of the enteric
Q41: If a particular reactant is involved in
Q42: For the exothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For
Q43: The rate of reaction for the decomposition