Multiple Choice
What is the half-reaction that occurs at the cathode during the electrolysis of an aqueous sodium iodide solution? 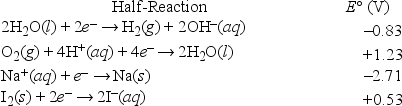
A) Na+ + e- →
B) O2(g) + 4H+(aq) + 4e- → 2H2O(l)
C) 2H2O + 2e- → H2 + 2OH-
D) I2 + 2e- → 2I-
E) 2I- → I2 + 2e-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: Given the following standard reduction potentials in
Q85: What is the purpose of a salt
Q86: When the following redox equation is balanced
Q87: A certain electrochemical cell has for its
Q88: _ occurs at the cathode in a
Q90: A SHE has the acid concentration of
Q91: Which equation is correct?<br>A) E = -(RT/nF)
Q92: Based on the data presented below, which
Q93: Based on the following electrochemical cell, which
Q94: What is the equilibrium constant at 25°C